
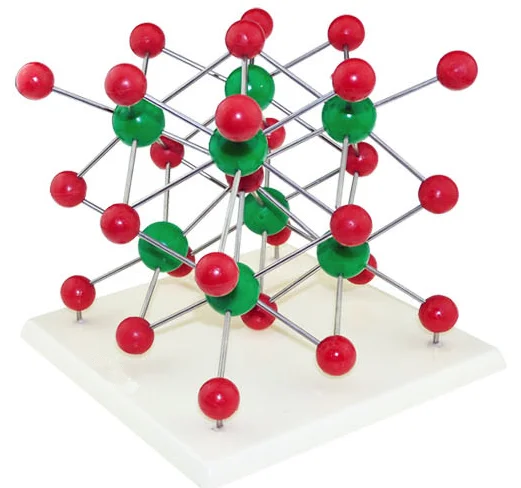

The LnCo(CN)6 frameworks’ lanthanoid and cobalt metal atoms are linked together by cyanide bridges. Just like the ends of a skipping rope get closer together when you vibrate it, these transverse motions cause the average metal-metal distances to decrease, thereby causing contraction of the crystal. As the temperature rises, these linkers start to vibrate more and more in skipping-rope-like transverse (sideways) vibrations. The lanthanoid and cobalt atoms are ‘bridged’ together by the cyanide (CN) ions. The key to the NTE is the networked structure of the materials. Once the water is removed from the crystal structure by heating, the NTE properties are activated. By the end of the video, they are about 2 mm in size and have started to merge together. LaCo(CN) 6♵H 2O is easy to grow, and forms nice hexagonal crystals. They can be easily crystallised from a solution as shown in this time-lapse video, recorded over the course of a few hours. The cyanide is tightly bound to the cobalt, so these materials aren’t particularly toxic, unlike potassium cyanide. Such a composite is immune to the undesirable effects of thermal expansion, from the buckling of railway tracks in the heat, to quartz-crystal clocks gaining or losing time when it’s too hot or cold.Ī related group of frameworks which also show NTE are the lanthanoid hexacyanidocobaltates, with chemical formula LnCo(CN) 6, where Ln can be any element from the lanthanoid row of the periodic table from lanthanum (La) to lutetium (Lu), Co is cobalt, and CN is cyanide. This unusual phenomenon is useful – if you combine an NTE material with a normal material in just the right ratio, you can make a mixture with zero thermal expansion. The record holder, which has the greatest rate of volume contraction upon warming over a wide temperature range, is single-network cadmium cyanide (we previously blogged about the double-network version). While most materials expand when heated, a few show the opposite behaviour, known as negative thermal expansion (NTE).


 0 kommentar(er)
0 kommentar(er)
